福建:药监局鼓励开展执业药师远程药事服务
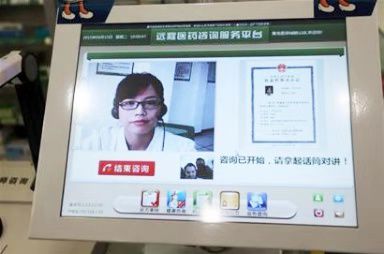

中国消费网福州讯 7月19日,福建省药品监督管理局对外公布了《关于鼓励通过信息技术开展执业药师远程审方的意见》(以下简称《意见》),明确了执业药师远程药事服务的相关要求。《意见》指出,支持药品零售连锁企业(以下简称连锁总部)利用现代信息技术开展远程处方审核,并为所属的药品零售连锁门店(以下简称门店)提供药事服务。连锁总部在福建省范围内开展远程药事服务,应符合《福建省鼓励发展药品连锁经营的意见(试行)》(闽食药监认〔2013〕154号)的相关规定。允许从事远程药事服务的执业药师注册到连锁总部,按规定接受继续教育。连锁总部根据门店处方审核高峰期的实际需求,可将注册在连锁总部的执业药师临时调配到门店帮助处方审核工作。执业药师应按照《处方管理办法》(卫生部53号令)的要求,及时详细核对处方,必要时与患者远程视频对话问询和答疑,审核后签署通过或不予通过的意见。处方审核相关工作信息、记录应当真实、完整并按规定期限保存备查。
Fujian: FDA encourages remote examination of licensed pharmacists
Fuzhou News, China Consumption Network, July 19, Fujian Provincial Drug Administration announced the "Opinions on Encouraging Licensed Pharmacists to conduct remote auditing through information technology" (hereinafter referred to as "Opinions"), clarifying the relevant requirements of licensed pharmacists remote auditing. The Opinion points out that it supports drug retail chain enterprises (hereinafter referred to as chain headquarters) to use modern information technology to carry out remote prescription audits and provide pharmaceutical services to their drug retail chain stores (hereinafter referred to as stores). Chain headquarters in Fujian Province to carry out remote auditing, should comply with the "Fujian Province to encourage the development of drug chain operations (trial)" (Fujian Food and Drug Supervision [2013] 154) of the relevant provisions. Licensed pharmacists engaged in remote auditing services are allowed to register at chain headquarters and receive continuing education as required. Chain headquarters can temporarily allocate licensed pharmacists registered in the chain headquarters to the stores to help with the prescription audit according to the actual needs of the peak period of prescription audit. Licensed pharmacists should check prescriptions in time and in detail according to the requirements of "Regulations on Prescription Management" (Decree No. 53 of the Ministry of Health). If necessary, they should have a remote video dialogue with patients to ask and answer questions. After examination, they should sign the opinions passed or not passed. The relevant information and records of prescription review shall be authentic and complete, and shall be kept for reference within the prescribed time limit.

